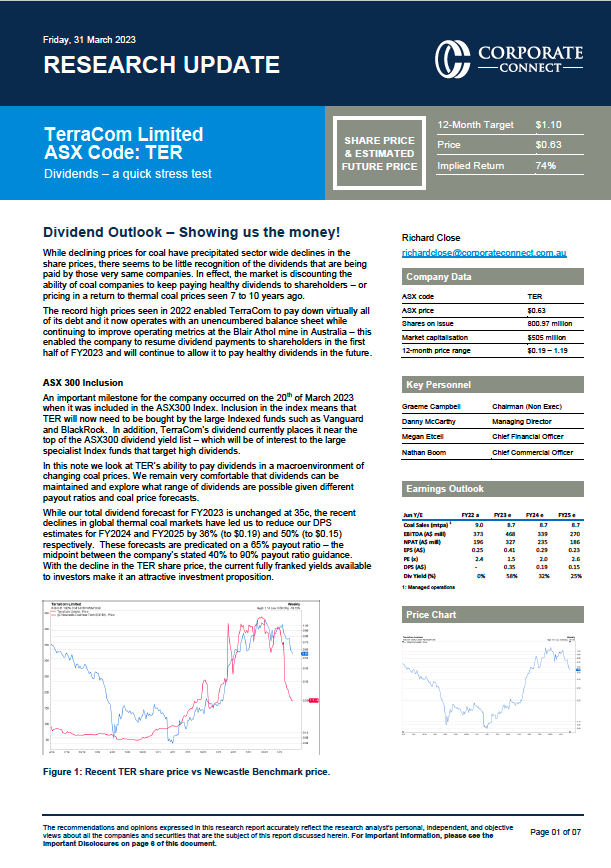
Mayne says it now has more than 30 pipeline products in the US, and more than 20 in Australia. It markets 30 different molecules globally – across 100 different presentations – and sells products in ten countries. Mayne also serves 125 contract customers.
A year ago, specialist pharmaceutical company Mayne Pharma Limited (MYX) was struggling with headwinds with its franchise for the Doryx acne treatment in the United States.
US sales of Doryx accounted for 16 per cent of Mayne’s revenue, but prescriptions for it were on the wane. Doryx’s Irish–based maker, Actavis, responded to the lower demand by cutting back manufacturing of the drug.
That strained the relationship, to put it mildly. Even though Mayne Pharma owned the patent – and had been manufacturing Doryx in Australia for three decades – Actavis owned the Doryx trade mark. Actavis also owned Mayne’s US marketing and distribution partner, Warner Chilcott, which it bought in October 2013. Mayne had lost control of its most important product – Doryx was generating margins of close to 70 per cent.
Mayne was also hit last year by disruptions to sales in the US of two of its other generic products, oxycodone and hydrocodone.
The problem was long–term distribution contracts, which did not have performance criteria, and in which Mayne had little negotiating power. The company saw itself losing revenue, with no control over its destiny. That was starkly shown in February when the half–year result (to December 2014) revealed a 15 per cent fall in revenue, to $59.5 million, a 23 per cent slide in EBITDA (earnings before interest, tax, depreciation and amortisation) to $14.6 million, and a 53 per cent slump in net profit, to $4 million.
The company could not let this continue, and it bit the bullet.
In February this year, Mayne Pharma struck a deal to buy the Doryx brand and assets in the US from Actavis for $US50 million, as part of a broader push into the market that involved setting up a new US division. In May it followed the Doryx deal by ending its distribution agreement with Mylan for its Oxycodone products – which have a $US1 billion market – taking control of the marketing under its own label. Mayne also took back from Mylan the distribution rights for methamphetamine tablets.
With the acquisition of the Doryx brand and distribution rights and the Oxycodone and Methamphetamine product franchises and the launch of these products under the Mayne Pharma label, the company had regained control of the distribution, sales and marketing – and the manufacturing – of these key products. The deals diversified Mayne’s US business into an integrated pharmaceutical business, with operations in generics, contract services and now specialty brands.
This was emphasised in August, when Mayne announced a US$65 million expansion of its US manufacturing operation (in Greenville, North Carolina), which was originally owned by Metrics Contract Services, a company Mayne bought in 2012 for US$120 million. Mayne has established at Greenville its US Specialty Brands Division, responsible for the marketing and distribution of branded products in the US. Greenville is the second manufacturing facility, behind the main plant at Salisbury in South Australia.
Mayne Pharma now has four business units:
• US Generic Products (GPD) – develops, manufactures, markets and distributes generic products in the US.
• US Specialty Brands (SBD) – responsible for the marketing and distribution of branded pharmaceuticals in the US.
• Metrics Contract Services (MCS) – provides contract pharmaceutical development services to third parties globally.
• Mayne Pharma International (MPI) – develops, manufactures, markets and distributes branded and generic products globally.
Mayne says it now has more than 30 pipeline products in the US, and more than 20 in Australia. It markets 30 different molecules globally – across 100 different presentations – and sells products in ten countries. Mayne also serves 125 contract customers.
42 per cent of revenue comes from generic products, 31 per cent from contract services and manufacturing and 27 per cent from branded products. 78 per cent of revenue is generated in the US, with 17 per cent coming from Australia and 5 per cent from the rest of the world.
The company says the decisions it took in the US over the last two years have “transformed” it. They did not take effect in time to show up in the 2014–15 result, with revenue sliding 2 per cent to $140.3 million, EBITDA falling by 10 per cent to $35.4 million and net profit plunging 64 per cent, to $7.8 million.
But the sharemarket has certainly been on–board with the turnaround story. Mayne Pharma started the year at 62 cents, but surged to $1.235 by May, on the back of the deals with the Doryx, Oxycodone and Methamphetamine product franchises. Mayne has slipped back to $1.022 as the market has digested the new business structure.
The company was upbeat in its outlook statement when delivering its FY16 result, and as the sharemarket looks forward to new product launches in FY16 and a longer–term pipeline – with the full benefit from re–absorbing the Doryx franchise to come – Mayne has seen earnings expectations rise. The analysts’ consensus expectation sees earnings per share (EPS) almost quadrupling this financial year – from 1.2 cents to 4.2 cents – and following that up with almost 50 per cent growth in FY17. That prices MYX on an undemanding 16.4 times expected FY17 earnings. The analysts also expect the dividend to be reinstated by FY17. And at $1.27, the analysts’ consensus target price implies a juicy capital gain of 25 per cent from present levels.