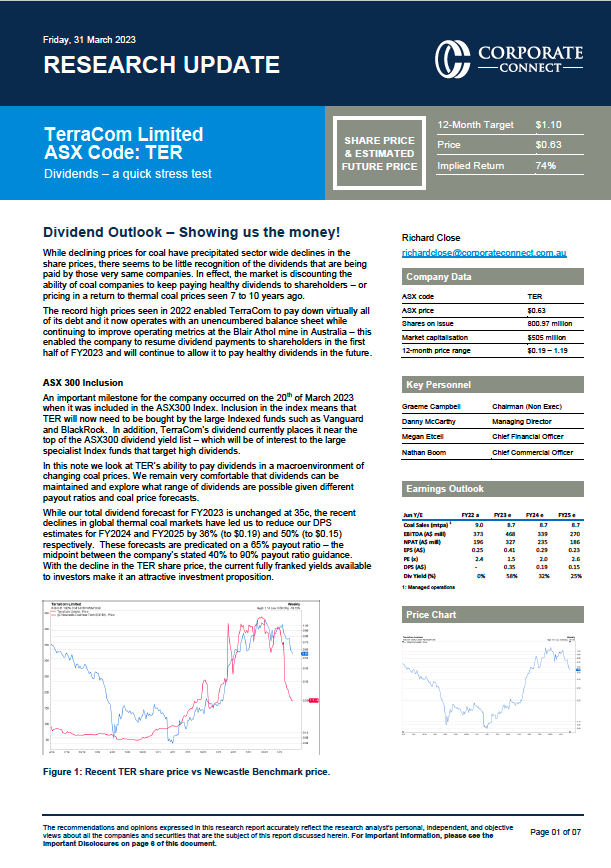
While Immutep boasts development candidates that have been licenced to Novartis AG (SWX: NOVN) in the area of cancer and GlaxoSmithKline plc (GSK; LON: GSK) in the area of autoimmune diseases, as well as an internal autoimmune disease candidate, IMP761 (which is close to commencing clinical trials), the company’s primary asset is efti. Efti is in several current, and many planned, clinical trials. While it could be argued that efti is furthest down the path toward a potential approval for the first-line treatment of hormone receptor positive, HER2 negative, metastatic breast cancer patients, with Immutep having announced phase III trial plans in its June capital raising presentation, there is a real possibility that the compound could obtain an accelerated approval from the FDA for the first-line treatment of head and neck squamous cell carcinoma (HNSCC) in combination with Merck’s pembrolizumab, based on the results of a single-arm phase IIb trial (ClinicalTrials.gov Identifier: NCT04811027) on which Immutep and Merck are formally collaborating.
Global leaders in the understanding and development of therapeutics that modulate Lymphocyte Activation Gene-3 or “LAG-3” which is a cell surface molecule which plays a vital role in regulating T cells. LAG-3 was discovered by our Chief Scientific Officer and Chief Medical Officer Dr. Frederic Triebel, putting Immutep at the forefront of immunotherapy drugs for cancer and autoimmune diseases.
IMM’s objective is to harness and strengthen the power of the body’s own immune systems through therapeutic intervention for the benefit of patients’ health. This is how immunotherapy fights cancer and autoimmune disease.