by Marshall Gordon and Jean Yu
Key Takeaways:
- The emergence of the COVID-19 Omicron variant shows the fight against the virus is ongoing and that COVID-19 could transition to an endemic virus.
- Existing COVID-19 vaccination, possible acceleration of general or variant-specific booster shots, and new antivirals leave the developed world well-positioned to handle the disease burden posed by variants.
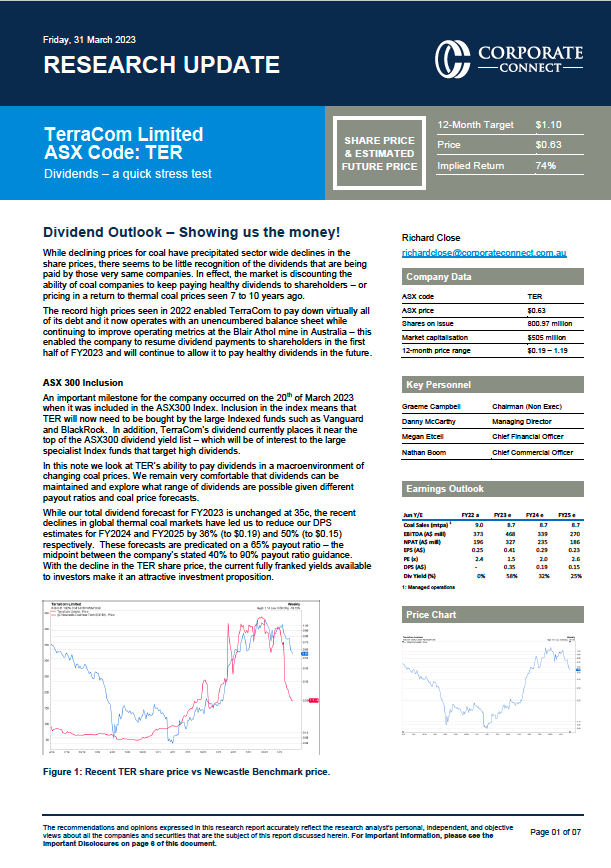
The emergence of the COVID-19 Omicron variant in South Africa on Black Friday generated a flurry of headlines as nations imposed new travel restrictions and equity markets suffered their worst day in over a year. While the challenge posed by this new variant has yet to be fully understood, the rapid detection and response of global health authorities shows how the fight against COVID-19 has evolved over the last 18 months. It is also changing the perception of COVID-19 from a limited time pandemic to the reality of long-term endemic transmission that will require ongoing scientific progress and public health action.
Based upon our preliminary understanding of the Omicron strain, the burden on developed nations’ health care infrastructure and hospital systems will most likely be more benign than previous variants and waves. In fact, given the growing array of tools to identify and manage COVID-19 infections, we believe each successive variant should have less of an impact in terms of severe disease and hospitalizations. Underdeveloped public health infrastructures and health care systems in emerging markets face a higher risk of an increase in cases and systemic strain. That said, in both developed and emerging countries, the imminent availability of antiviral pills has the potential to reduce the impact of the Omicron or subsequent strains.
With the emergence of the latest Omicron variant, companies engaged in vaccine development, antiviral treatments and manufacturers of medical diagnostic equipment have seen increased demand for their services. The last 18 months has allowed vaccine manufacturers, specifically those specializing in mRNA vaccines, to develop the infrastructure to combat new variants quickly and cost-effectively. Pfizer and Moderna, leaders in vaccine manufacturing, have commented that the development of an Omicron-specific vaccine could take as little as four to six months to bring to market, should one be necessary.
Another means of treatment, COVID-19 antiviral pills, have been developed to target different viral proteins than the ones targeted by vaccines, allowing them to retain their full efficacy against Omicron and other future mutations of the virus. Due to strong demand, Pfizer and Merck announced their intention to ramp up manufacturing to 30-80 million courses each by the end of 2022. Additionally, the ability to allow other companies to manufacture the antiviral pills on their behalf allows both companies to respond to any widespread outbreak quickly and effectively.
While we will await further clarity on the challenge posed by the Omicron variant, the need to safeguard the health of large populations continues to create opportunities that leverage the innovation of biopharmaceutical and lifescience tools manufacturers. The emergence of Omicron serves as a reminder that the pandemic is not over, and we remain optimistic about the long-term opportunities this creates for companies that develop new vaccines and medicines to fight the virus and diagnostics to diagnose and track it.