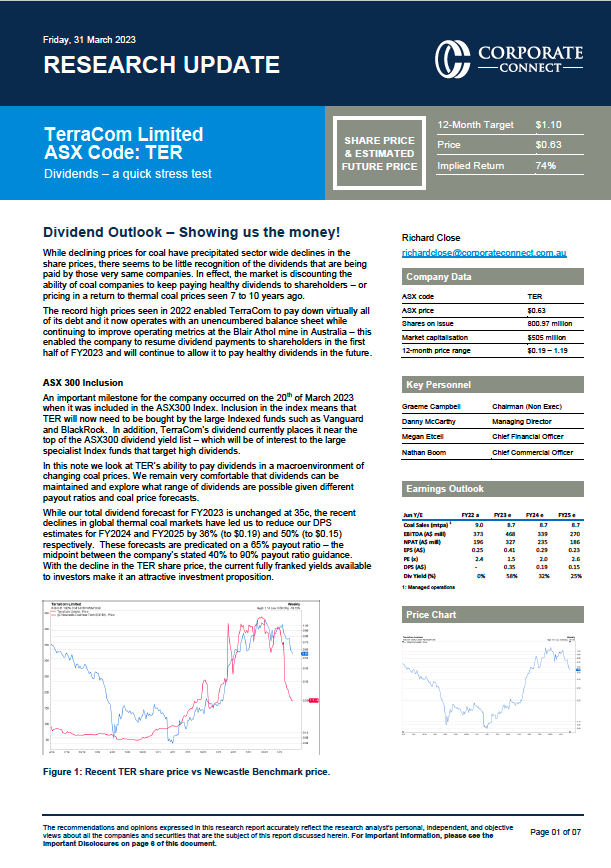
As an analyst who follows biopharma companies, I take an interest in the US Food and Drug Administration (FDA), while as an American/Australian, US politics, particularly, in the days of Trump, is also an interest. Then, there is an ASX-listed cellular therapy company called Mesoblast (ASX:MSB), which I have followed pretty much since it listed in 2004.
In mid-August this year, I spent a torrid 3-days reviewing nearly 300 pages of what are termed briefing documents on Mesoblast’s most advanced therapy, remestemce-L, for use in treating a condition which occurs in patients who have had a bone marrow transplant, in this case specifically for use in children. The briefing documents were posted by the FDA two and a half days before it convened what is called an advisory committee meeting, commonly called an AdCom. During the AdCom, the FDA and Mesoblast debated the evidence for approval of remestemcel-L in front of a panel of independent experts.
Understanding the briefing documents is important because it is from them that the analyst must decide how the meeting, which is publically broadcast, will go for the company. Moreover, there isn’t much middle ground after the AdCom, with the company’s stock price likely to move appreciably afterward.
While these meetings can be complex, as was Mesoblast’s, the last item on the agenda usually determines how the market percieves the company’s performance. This last item is a question that asks the experts to vote. There can be other voting questions but the last one is invariably the big one. This question can take quite a few forms but can be generalised to, “based on the evidence presented, should the FDA approve the therapy/drug in question?” Remestemcel-L received nine“yes” votes panelists and one “no”. Mesoblast‘s shares were up an appreciable 39.1% for the trading day.
I never got a chance to put out a report out in between the release of the briefing documents and the AdCom. I did, however, make the right call. In short, I told the dealers where I worked at the time, those men and women who advise investors on what stocks to buy and sell, that Mesoblast should get a positive vote on the last question and that whatever else that happens during the meeting will be drowned out by that vote.
The thrill of being right doesn’t last long. The market always looks forward and the FDA is not bound by the discussion or vote(s) during an AdCom when it makes its final decision on whether to approve a therapy. The FDA’s final decision generally comes 4-6 weeks after the meeting. Moreover, you have just learned a whole lot about what was actually in the company’s application for approval to the FDA, since the FDA follows the AdCom vote about 80%, the game isn’t over yet. You then have to rule out some theories you had developed about the company’s future, give others greater thought and, as in this case, I added one, as I thought about the company as a whole and with reference to the FDA’s decision on remestemce-L, due on or before Septemer 30th.
In a recent article, I read about President Trump standing proudly as the FDA Commissioner announced an Emergency Use Authorisation (EUA) for a therapy for COVID-19 called convalescent plasma.The FDA is allowed to issue EUA’s, which allow the legal use of an unapproved therapy, in cases of severe health emergencies. A similar scene played out in late April, when the head of the US National Institutes of Allergy and Infectious Diseases (NIAID), Dr Anthony Fauci, announced positive results with Gilead Sciences’ remdesivir in treatng patients with severe COVID-19. Trump was beaming. Days later, the FDA announced an EUA for remdesivir.
Both decisions were highly contentious and negative responses from scientists and physicians, strongly outweighed the positive. It was alleged Trump was improperly influencing FDA decision making. Previous presidents have, at least openly, left the FDA to make decisions based on science and agency rules. Trump appears to be an exception and his past acts fueled the view. With that, another variable entered into to my thoughts on Mesoblast’s future.
Remestemcel-L is currently in a very well designed, 300 patient, US government-funded trial where it is being used to treat COVID-19 patients with acute respiratory distress syndrome (ARDS). ARDS is essentially an over-reaction of the immune system to an insult, like SARS-CoV-2 infection, the virus that causes COVID-19. ARDS is the ultimate cause of death in most COVID-19 patients.
The results from the first 90 patients through this trial are due any day now. Given Trump is severely behind in polling and his handling of COVID-19 seems to be a major factor for that, the results from this trial become much more important than they might normally be for Mesoblast and certainly for Trump.
It seems plausible that Trump could push the FDA to build up remestemcel-L, even if the results from this first analysis are only mildly positive. It would give Trump what he needs, more evidence that his way was the right way to deal with the pandemic. If Mesoblast knocks it out of the park, Trump may even claim national Martin Luther King, Jr Day will be replaced with Mesoblast Day.
There seems to be two likely outcomes of such a scenario.
The first is that remestmcel-L would likely be granted an EUA for COVID-19 associated ARDS. This could fast-track remestemcel-L for ARDS associated with many other conditions, from infuenza to phyical lung injury. The fact that ARDS is highly lethal, with no approved treatment means three more things. Relatively easy passge through the FDA, a very high price for the therapy and access to a large market with no other competitors.
The second is that it would seem almost impossible for the FDA to deny remestemcel-L approval for the current indication it is seeking it for, when just weeks before it granted an EUA for COVID-19 associated ARDS.
To mimic Dr. Seuss badly, oh the thinks and analyst must think.