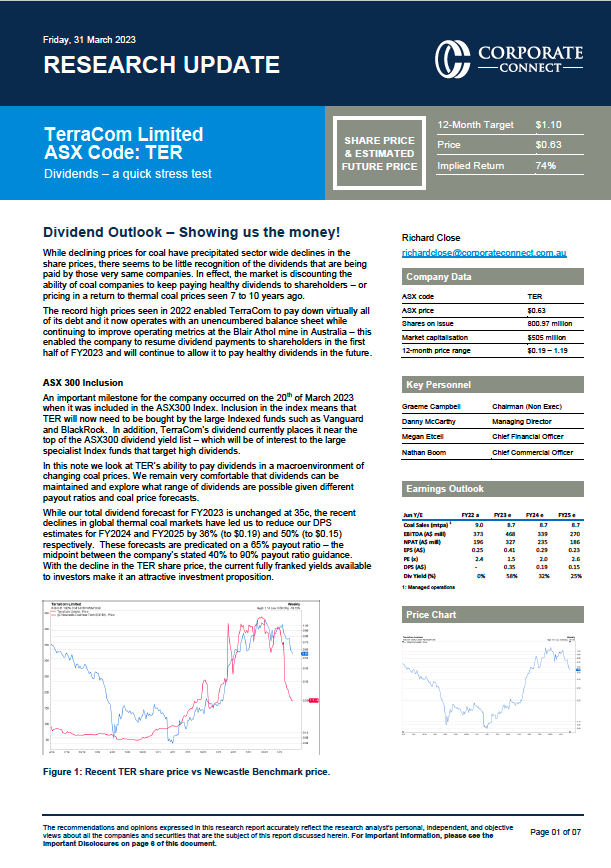
Two announcements from drug developer Antisense Therapeutics have raised hopes of an expedited path to market for its novel candidate to treat Duchene muscular dystrophy (DMD), a genetic muscular disorder that affects only boys and is regressive, fatal, and poorly treated.
On September 30 the US Food & Drug Administration (FDA) awarded Antisense ‘rare paediatric disease designation’ (RPDD) for its lead candidate, ATL1102.
An RPDD means the company potentially is eligible for a rare paediatric disease review voucher – a valuable right that can be on-sold to another drug developer.
Two days later the company unveiled clinical data from a local phase 2 trial that favorably compared the results of an ATL1102-treated cohort with overseas data from a matched group of DMD boys treated with corticosteroids.
For Antisense chief executive Mark Diamond, the events herald the most exciting period for the company since he clocked on as CEO almost 20 years ago.
In particular, he believes a planned follow-up phase 2b trial in Europe could be enough to win Continental approval if the results emulate the phase 2 data.
“This study could turn out to be a pivotal trial,” Mr Diamond says. “In other words, if we get the requisite data showing significant clinical improvement in upper limb function we could submit for European approval.”
Affecting about one in 3500 males from birth to 18 years, DMD is caused by a mutation in the muscle dystrophy gene, leading to severe progressive muscle loss and premature death. The current standard of care, corticosteroids have limited efficacy and significant side effects when used continuously, as required.
In-licensed from the Nasdaq-listed antisense drug developer Ionis Pharmaceuticals, ATL1102 is an antisense inhibitor of the VLA-4 protein, also known as CD49d, a target for the treatment of multiple sclerosis.
ATL1102 inhibits the inflammation caused by the lack of the protein dystrophin, which erodes muscle function.
DMD is the biggest fatal genetic disease but is thankfully rare, affecting about 48,000 boys in the US and Europe.
While Antisense announced the ‘top line’ phase 2 results in May this year, the details of the comparison of the phase 2 data to the matched overseas control group were outlined to peers – virtually of course – at the recent World Muscle Society’s annual congress.
Carried out at the Melbourne Royal Children’s Hospital neuromuscular centre, the trial enrolled nine non ambulant (wheelchair bound) patients. The boys were treated with ATL1102 over six months, with all but one remaining on the corticosteroids.
The boys’ muscle function was then compared with the recorded results from 20 boys treated with corticosteroids over the same six month time period. This data was held by leading DMD expert Professor Eugenio Mercuri, of Rome’s Catholic University.
In results dubbed as statistically significant, the ATL1102-treated boys performed better on the muscle function assessment score after 24 weeks.
The ATL1102 patients achieved a mean improvement of 0.89 on the standard measure, the Performance of Upper Limb Function (PUL2) test. Put in context, the so-called Rome Cohort saw an average decline of -2.0. Also 78 per cent showed no change or an improvement, compared with 33 per cent for the control group.
“This means the [ATL1102] boys aren’t losing function that won’t be regained – that’s the critical point,” Mr Diamond says. “Once these boys become non-ambulant and lose this function it is not retrievable and they are on a linear steady decline over time.”
Mr Diamond said that, if replicated in the planned phase 2b trial of about 100 patients treated for one year, this improvement should be enough to get the drug approved by the European Medicines Agency.
As for the RPDD status awarded by the FDA, a follow-up voucher grant would confer a rich means of non-dilutive funding if Antisense opted to sell the asset.
As the name suggests, the paperwork enables applicants to request a fast track review, which can hasten the approval time by between four to six months.
While this might not seem like much, an extra few months’ on market can mean billions of dollars of extra sales for a blockbuster drug.
That’s why the vouchers – dubbed golden tickets – have been on sold for between $US68 million and an eye-watering $US350 million.
“The designation doesn’t guarantee a voucher, you still need to meet the eligibility criteria,” Mr Diamond says. “But having the designation gives you confidence the FDA believes the disease you are treating meets their conditions.”
Intriguingly, Antisense is the second ASX-listed drug developer to win RPDD designation in the space of weeks. In early August Kazia Therapeutics was bestowed the ‘honour’ for its drug candidate for a rare childhood brain cancer called Diffuse Intrinsic Pontine Glioma.
US drug developer Sarepta Therapeutics won FDA approval for two DMD drugs – and a voucher which it on-sold for $US125 million in 2017.
The Nasdaq-listed Sarepta is well known to Antisense because its former executive chairman William Goolsbee sits on the Antisense board.
As well as having a different mechanism of action, Sarepta’s two drugs only work for a subset of 20 per cent of patients with two genetic mutations.
But just as Sarepta’s drugs are used in combination with corticosteroids, Mr Diamond says ATL1102 potentially could be used in conjunction with Sarepta’s therapies, to treat both ambulant and non ambulant boys.
“All boys with DMD at some stage will need to have the inflammation treated,” he says.
As if Antisense doesn’t have enough on its regulatory plate, it’s also applied to both the FDA and the European Medicines Agency for ‘orphan drug’ designation, which pertains to diseases with no effective treatments.
An FDA decision is imminent.
“Orphan drug status brings additional benefits, like ten and seven years of market exclusivity in Europe and the US respectively and waiving of certain registration fees which can be quite substantial,” Mr Diamond says.
With $4.1 million in the bank as of June 30, Antisense is well placed to fund the regulatory aspects leading up to the phase 2b trial, but the trial itself would necessitate further capital.
“We’ve got a bit of time to think over the options,” Mr Diamond says. “We also have pharma company interest in the program, even though we have not been actively out there seeking it.”
The slew of upbeat news means that Antisense shares are now trading close to five year highs – even though the October 2 trial tidings coincided with reports of Donald Trump’s flirtation with the coronavirus which spooked the broader market.
With the pending FDA ‘orphan’ drug decision and the European trial plans unfolding, investors might not have to wait long for more potential catalysts.
On the latter, the company expects to lodge a clinical trial application in the first half of 2020 – with first patient recruitment shortly thereafter.
Mr Diamond dubs the recent developments as “historic milestones” for Antisense, which sprung from the first ASX-listed biotech, Circadian Technologies.
“They are substantial de risking events for our ongoing development,” he says. “They also elevate the quality and significance of our data and bring hope to the young victims of such a cruel disease.”